Summary
The developed 3D hybrid bioprinting system allows printing at temperatures up to 250°C and solventless coating of the surface of the printed polymer implant material almost simultaneously in one single machine. It further has the capability to print materials including polymers, polymer blends and composites with graded properties in porosity, mechanical stability and bioactivity. 3D products in the focus of the FAST project are affordable biodegradable bone scaffolds. Target fields of application of these scaffolds include treatment subsequent to bone trauma, tumor, infection, non-union after (large-)fracture where it is the aim to offer an advanced alternative to existing methods with
- improved osseointegration of the bone scaffolds
- enhanced suppression of post-surgery infections
- optimized implant lifetime
- reduction of the number of revision surgeries
in order to increase patient comfort and reduce the load for the health provision system.
Result description
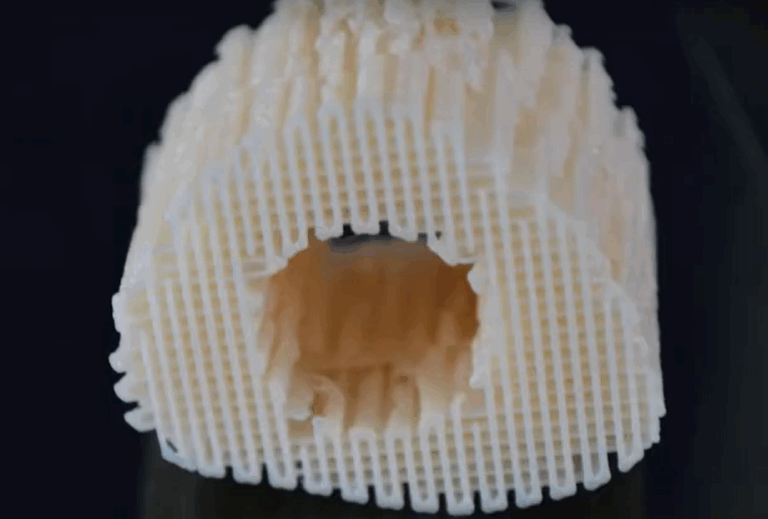
A continuous thermoplastic composition gradient print head has been developed and integrated together with a plasma jet in a bioscaffolder unit. The plasma jet allows solventless coating of the as-printed material e.g. with cell-growth promoting factors at atmospheric pressure. The system has already been extensively tested in the printing of scaffold structures consisting of poly(ethylene oxide terephthalate) (PEOT)/poly(butylene terephthalate) (PBT) polymer blend composites and at temperatures of more than 200°C. These are promising materials for bone regeneration due to the combination of mechanical properties, biocompatibility and biodegradability as well as the possibilities for the integration of bio-active molecules and cell-growth controlling surface chemistry they offer. For instance, osteogenic differentiation and matrix mineralisation has been shown to be enhanced in polymer composites containing hydroxyapatite fillers. With scaffold materials containing fillers carrying suitable antibiotic substances cell viability could be married with antimicrobial activity of the scaffolds.
Addressing target audiences and expressing needs
- To raise awareness and possibly influence policy
- Grants and Subsidies
- Other blended financing
- Help in technical expertise
- Use of research Infrastructure
- Collaboration
- Contacts to medtech companies with expertise in the field of medical regulation and contacts to regulatory bodies such as the FDA as well as knowlegde and experience in the commercialisation of medtech products.
- Contacts to orthopaedic surgeons at university hospitals
- Funding sources for further development work, and in-vitro/in-vivo testing
- Public or private funding institutions
- EU and Member State Policy-makers
- Research and Technology Organisations
- Academia/ Universities
- Private Investors
R&D, Technology and Innovation aspects
3D hybrid printing system demonstrators are available combining 3D printing process and plasma polymer deposition. New polymer blend based materials with nanofillers increasing mechanical stability or equipping the scaffolds with antibacterial properties are available. In-vitro test series were performed successfully. Animal testing results are analysed.
Result submitted to Horizon Results Platform by ABALONYX AS