Result description
In SALBAGE project, the University of Southampton has demonstrated the use of electrolyte additives as catalytic species to facilitate the kinetics of the reactions in aluminium-sulfur batteries, which are otherwise very sluggish. The catalytic species can improve performance metrics such as capacity, rechargeability, energy density and energy efficiency, thus paving the way towards the commercialization of aluminium-sulfur batteries as a promising novel beyond lithium-ion technology that is made of cheap and highly abundant materials such as aluminium and sulfur.
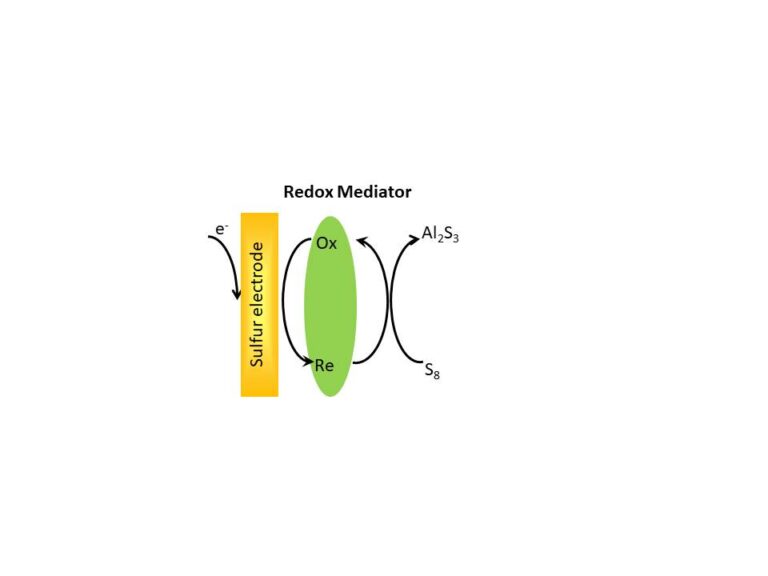
Redox mediators have been added as electrolyte additives in aluminium-sulfur batteries to facilitate the electrochemical reactions. A significant improvement in the rechargeability of the cells has been demonstrated, with a decrease in the charge voltage by ca. 0.2 V. A variety of redox mediators are currently under investigation. To the best of our knowledge, this is the first time that redox mediators have been incorporated in a multivalent metal-sulfur battery, and opens a new venue of investigation of new materials that could produce step changes in performance while maintaining the intrinsic benefits of multivalent metal (e.g. aluminium)-sulfur batteries such as low price and high abundance of the battery components.
Addressing target audiences and expressing needs
- Collaboration
To collaborate with other institutions and/or industries willing to advance in the research and implement the results.
- Public or private funding institutions
- Other Actors who can help us fulfil our market potential
- Research and Technology Organisations
- Academia/ Universities
R&D, Technology and Innovation aspects
The redox mediators have been tested on a small, lab scale electrochemical cell containing an aluminium anode and a sulfur cathode.
Result submitted to Horizon Results Platform by UNIVERSITY OF SOUTHAMPTON