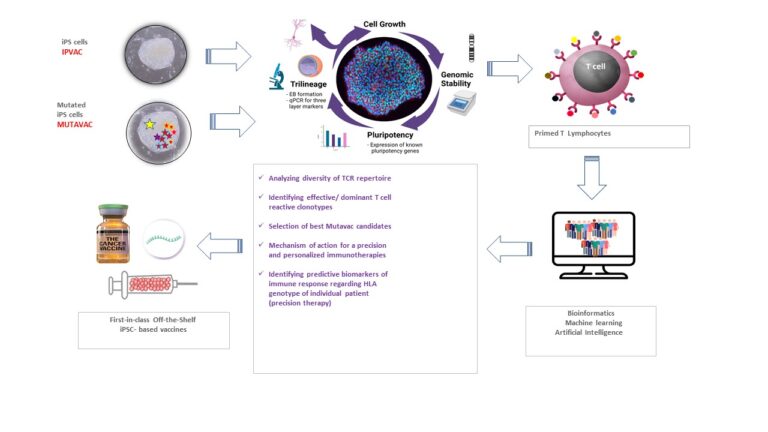
Result description
During MUTAVAC project (EIC pathfinder), IPSIRIUS has 1- established the proof of concept of successful mutagenesis of human iPSCs 2-Discovered novel clinically useful mutanome reproducing human cancer and leukemia 3- Generated of an immunogenic potency model using mutagenized iPSCs and dendritic cells able to stimulate T-cell response, 4- validated the composition of the final products “IPVAC-Mut” able to deliver embryonic tumor specific neoantigens shared with aggressive cancers. MUTAVAC program will also identify patients expressing the tumor neoantigens targeted by IPVAC-Mut. Results of MUTAVAC project will allow to continue the development of IPVAC and IPVAC-Mut technology with the goal of providing patients with innovative therapeutic cancer vaccines based on a disruptive and unique technology. The next step will aim to transfer the technology into a pre-GMP manufacturing environment before producing a GMP-grade batch of the products and preparing a first-in-man for patients resistant to conventional therapies.
Addressing target audiences and expressing needs
- Grants and Subsidies
IPSIRIUS is looking for funds to produce a large scale batches of mutagenized and unmutagenized IPS cells that will be used to produce IPVAC and IPVAC-Mut for future phase I/II clinical studies. SNBTS/Edinburgh will bring support as CDMO for the development of iPSC manufacturing in cGMP conditions. IPSIRIUS will secure this industrialization phase by gathering all the expertise required (quality, regulatory and technical skills) thanks to the CDMO and the Miltenyi Biotech (Prodigy) technology.
- Public or private funding institutions
R&D, Technology and Innovation aspects
IPSIRIUS has reached TRL4 and demonstrated the technology in an,d R&D environment.
IPSIRIUS now ambitions, with the support of a CDMO (Contract Development Manufacturing Organisation) to achieve TRL5 by translating the solution into a pre-GMP environement and then TRL6 with the GMP manufacturing environment and development of a first Phase I/II clinical trial with an escalating dose of IPVAC, with support of a CRO (Contract ResearchOrganisation) who will be in charge along with IPSIRIUS to conduct clinical trials (medical writing,monitoring, data management, and statistical analysis).
IPSIRIUS is committed to developing a portfolio of engineered immune cell products using our proprietary induced Pluripotent Stem Cells (iPSCs) biotechnology platform. Our objectives are to:
- Generate the first proprietary line of mutagenized and unmutagenized iPSCs in compliance with GMP standards.
- Create value by advancing projects to Phase I/IIb clinical trials for NSCLC patients.
- Establish partnerships and licensing agreements with pharmaceutical companies, potentially engaging in co-development during Phase II.
IPSIRIUS has developed a robust and replicable technology to produce standardized, off-the-shelf, pan-cancer vaccines (IPVAC and IPVACmut) targeting epithelial cancers with high metastatic risk. Our product leverages Nobel Prize-winning induced Pluripotent Stem (iPS) cells, modified through proprietary pharmacological treatments to share multiple neo-antigens with metastatic tumor cells. When lethally irradiated iPS cells are administered to mice with aggressive tumors, these neo-antigens elicit a cytotoxic and memory immune response against a wide array of neo-antigens common to many agressive cancers. This response has consistently and significantly inhibited tumor growth, prevented metastatic spread, and increased survival rates in various cancer models (breast, lung, pancreas). The application of these vaccines will be guided by the genomic profile and genetic classification of the targeted cancers.
IPSIRIUS is developing two cancer vaccines based on iPSCs biotechnology:
- IPVAC: Utilizing iPSCs, this vaccine is intended for a multi-cancer (pan-cancer) indication, with an initial clinical focus on non-small cell lung cancer (NSCLC).
- IPVAC Mut: This next-generation immunotherapy product uses mutagenized iPSCs and is designed for specific, highly mutated cancers, including NSCLC.
Additionally, IPSIRIUS will design and manufacture its own iPS cells (autologous, allogenic, or universal) for customers. These cells will be available for partnership or licensing agreements with pharmaceutical companies, which will oversee further development and commercialization.
- Canada
- Global
- Europe
- North America
- Asia
Result submitted to Horizon Results Platform by IPSIRIUS